
The Nitrogen Cycle
Nitrogen, ammonia, nitrites, nitrates
Nitrogen (N) is an essential element of animal and plant tissues. Nitrogenous molecules, coming from animals (fish excrement), vegetables or surplus food, are transformed into ammonia (NH3 or NH4+), which is very toxic to fish. This phenomenon also happens in nature, but the volume of water is very large compared to the number of fish, so the concentration of ammonia is very low. In aquariums, the volume of water is small and the concentration of ammonia can become dangerous under certain conditions.
The transformation of ammonia into nitrites and from nitrites into nitrates is called the Nitrogen Cycle...
NOTE: in order to preserve the names that you will find in all the documents in English, I preferred in this document to use the name nitrites/nitrates instead of nitrogens/nitrogens, in English both names are correct and used.
In the presence of oxygen in the water, ammonia is transformed by bacteria (nitrosomonas) into nitrites* (NO2-). They are toxic at values higher than 0.3 mg/l.
Nitrites, in the presence of oxygen, are in turn transformed by other bacteria (nitrobacter) into Nitrates (NO3-). Nitrates are less toxic for living things in the aquatic environment and for plants they are even a strictly necessary nutrient. Nitrates are the final link in these nitrogen transformations, accumulating over time. The maximum concentration of nitrates is 100 mg/l (it is recommended not to exceed the value of 50 mg/l).
This chain of transformations is called the "Nitrogen Cycle".
The Nitrogen Cycle

All the elements that participate in this cycle (oxygen, bacteria, pH, temperature, plants, primary organic matter from which ammonia comes, etc.) must be kept in balance.
At the beginning of the process, in a new tank, the number of bacteria is low. Their multiplication is done slowly, as the amount of ammonia and respectively nitrites in the water increases. In the beginning, the bacteria that decompose ammonia into nitrites develop and after the concentration of nitrites exceeds a certain value, the bacteria that transform nitrites into nitrates begin to multiply.
After a period of time, the bacterial colonies become sufficiently developed to convert all the ammonia into nitrites and the nitrites into nitrates.
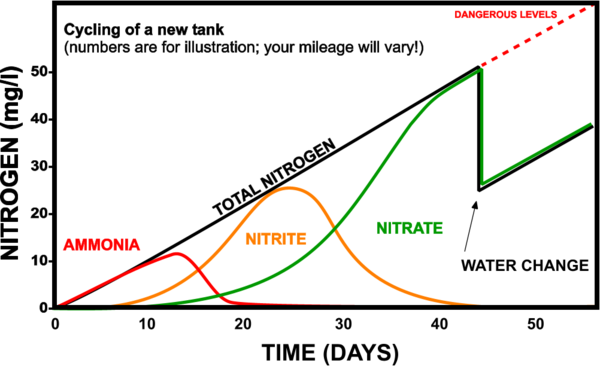
During this process, because the number of bacteria is small, ammonia and nitrite concentrations often exceed the maximum values, which leads to many disasters.
The "new aquarium syndrome" would translate into the fact that beginners populate the aquarium as soon as they fill it with water. Because of the high levels of ammonia and nitrites at the beginning of the nitrogen cycle, disasters occur (fish die). The first temptation of the beginner is to immediately change a large amount of water (most of the time all the water), to wash the filters very often and/or to introduce drugs into the aquarium... All this leads to the destruction of the bacterial colonies that had just started to form and... everything starts over...
To avoid such disasters, we recommend that you follow a few rules when starting a new aquarium:
- no fish will be introduced into the aquarium during the first week. During this period, fish food for 1-2 fish will be introduced into the aquarium.
- After the first week, the first fish will be introduced (2-4 depending on the size of the aquarium). They must necessarily be of very resistant, unpretentious species and preferably cheaper! Usually they are from the family of "sanitaries".
- After another 3 weeks, we can consider that the aquarium is sufficiently colonized with bacteria to be able to fully populate it with fish.
However, I recommend periodic tests to determine the exact values of ammonia, nitrites and nitrates in order to keep the nitrogen cycle and the state of the aquarium under control.
The hardest thing for an aquarist is to sit and wait 30 days next to an aquarium without fish... Remember that this effort is worth it and you will save another weeks of work rebalancing the aquarium or losing some fish specimens valuable or that you care about.
Nitrogen Cycle Control
In general, we can consider that in this system one of the parameters that is very easy to control is ammonia, respectively the primary sources from which ammonia appears. Keeping an optimal number of fish per volume of water in the aquarium and avoiding overfeeding them are the ways most within our reach to keep the balance.
Also, in a well-planted aquarium, it is easier to keep this balance, as the plants consume nitrates.
Water oxygenation is another factor that favors the activity of bacteria. Through regular water changes, excess food, vegetable remains and other organic matter and mainly excess nitrates are removed from the aquarium (in other words, we reduce their concentration).
The bacteria that carry out the transformations in the nitrogen cycle are found everywhere in our environment (water, air...). All that remains for us to do is to create an environment conducive to their development. They develop, in general, on the surface of any object in an aquarium (windows, stones, filters, etc.) and very little in the water. To fulfill its role, it must have a very large surface area and come into contact with a large amount of oxygenated water. For these reasons, the best environment for the development of bacterial colonies are filters (in them a slow water circulation favors the process).
Another very important factor in mastering the Nitrogen Cycle is the periodic replacement of a quantity of water. Through this action, excess nitrates are removed and micronutrients necessary for plant growth are introduced into the aquarium, together with the fresh water. The amount of water
The cycle time of a new aquarium can be reduced by several methods:
The use in the first period (without fish in the aquarium) of chemicals containing ammonium to force colonization with bacteria (see also the article Cycling without fish).
The introduction of special products that contain bacteria (in our country the best-known product is Cycle, but there are others). I know of no notable achievements using these products...
Introduction to the new aquarium of colonized environment (sand, filters, etc.) from cycled aquariums. This method is not recommended from my point of view because together with them unwanted parasites and/or bacteria, algae, etc. can be introduced into the new aquarium. Also, without a minimum required amount of ammonia or nitrites, these bacterial colonies will shrink to the equilibrium level...
The use of natural plants. There are some species, such as Ceratopteris and Hygrophila, which are very useful especially when starting a new aquarium because they are very resistant and consume a lot of nitrates.
As shown, a very important role is the development environment of the bacteria. The cheapest (and at the same time very effective) is the sponge filter connected to an air vibrator. This is improperly called a "filter" because its role is a development environment for bacteria (this filter can also be called a "biological filter") and not mechanical water filtration - by filtration most people understand mechanical filtration, meaning the retention of impurities. You have to keep in mind that by washing it, you reduce the number of bacteria or even destroy them all (washing is still necessary because by depositing impurities the sponge gets clogged and water circulation is prevented). For this reason, I recommend using two filters and washing them in turn and only when necessary. For a proper cleaning of the sponge, I recommend a first wash in a 30% food vinegar solution, followed by a long wash in running water. Attention: vinegar completely destroys bacteria and is used when you have two sponges. If you only have a sponge, washing must be quick and only in water, preferably in water without chlorine that destroys bacteria.
Other filters contain "biological" areas necessary for the development of bacterial colonies. They consist of pieces of sponge, sand, ceramic rings, etc.
In a fully cycled and balanced aquarium, the destruction of bacterial colonies by washing the filters will not lead to a very high increase in ammonia and nitrite values because bacteria are found, as I said before, on all surfaces of the objects in the aquarium. These will help to quickly restore the bacteria colonies in the washed filters.
CAREFUL:
Colonies of bacteria that help with the nitrogen cycle are destroyed by most drugs and chemicals used in aquarium treatments. This is another reason (very little presented or even omitted in the specialized literature) to use quarantine aquariums in which to do the treatments. Keep this in mind every time you do a treatment directly in the aquarium. Maybe the "new aquarium syndrome" appears even in stable aquariums for a long time, all the more so as the fish are weakened by the disease and the treatment itself... After such a treatment use all the methods at your disposal to reduce the amount of ammonia/nitrites: daily water changes for 1-2 weeks, strong aeration, speeding up colonization with bacteria (sponges from other cycled pools – with all the related risks), etc.
Another mistake I've seen many novice aquarists make is to use terrestrial plant fertilizers in the aquarium. These fertilizers have as basic components the N-P-K triplet (Nitrates, Phosphates, Potassium). On most of the packaging of these products is written a series of 3 numbers (for example: 2-2-50 or 10-20-40) which represent the concentration of these substances in the product. To those exposed above in the article, from which you realize what the introduction of excess nitrates represents, there is also the fact that phosphates are also lethal in very low concentrations. I think the painting is complete and does not require comments...
Fertilizing solutions for aquariums do not contain N-P fractions (or the amounts are very small).
Another alarm signal I raise about the water used. Many of these contain nitrates (and phosphates) in smaller or larger amounts depending on the source of the water used. Even drinking water has a low content of nitrates (accepted in the regulations in force regarding the quality of drinking water), in wells and running waters this content is much higher (keep in mind that enormous amounts of N-P-K fertilizers are used in agriculture! !!).
Ammonia
The maximum ammonia concentration allowed in aquarium water depends on the pH and temperature of the water (and the time this concentration is maintained). The table below shows the maximum values allowed in mg/l (maintaining the fish at these values becomes lethal in a few hours):

It is observed that in waters with a low pH and temperature, the maximum allowed concentration of ammonia is much higher than for waters with a high pH and temperature.
